Abstract
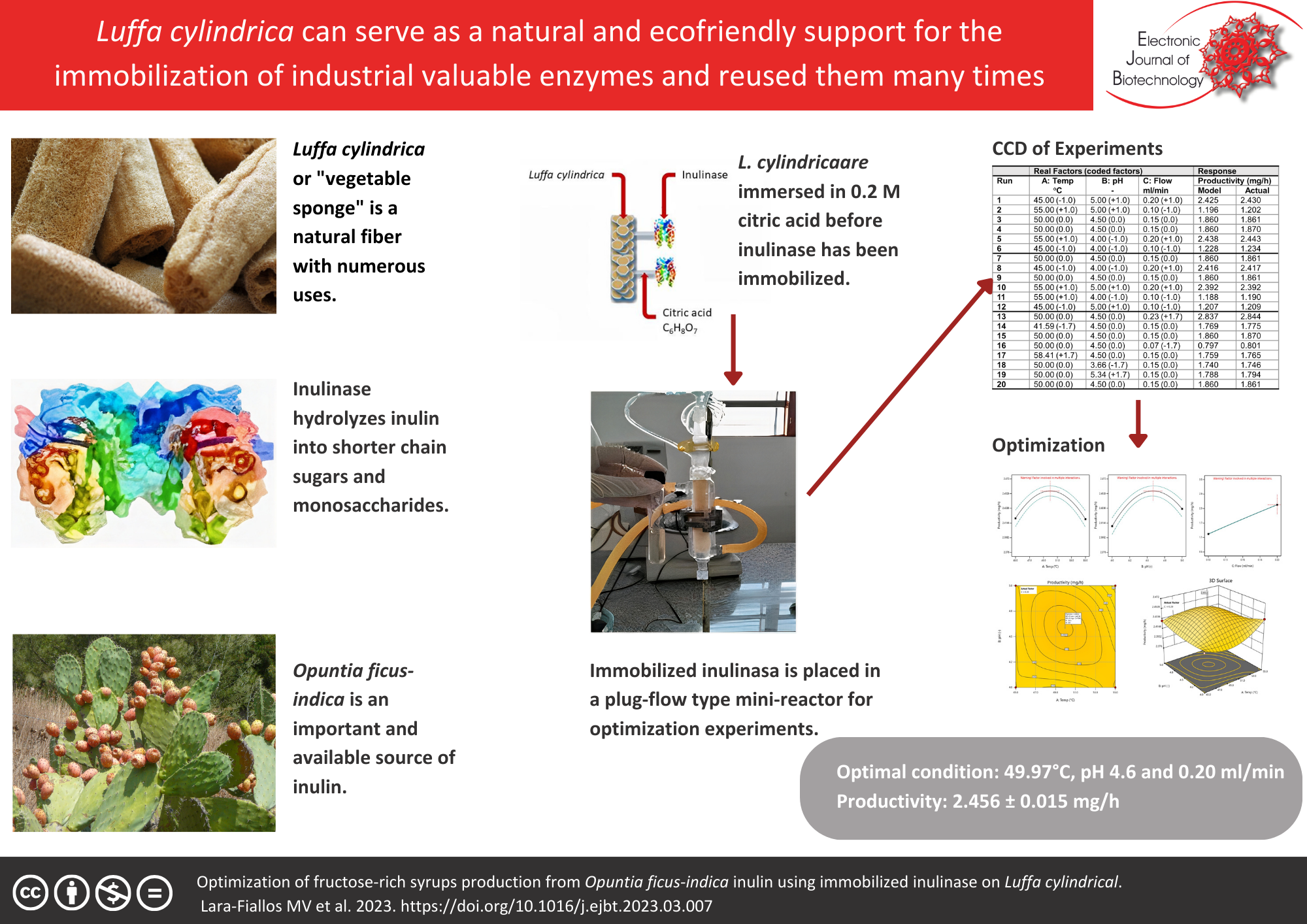
Background: Luffa cylindrica has numerous domestic and industrial uses. For example, this natural fiber supports immobilization through the covalent bonding of an inulinase. In addition, fructose syrup from crude inulin obtained from prickly pear (Opuntia ficus-indica) can be obtained in a plug-flow mini reactor with this immobilized inulinase on L. cylindrica.
Results: A central composite design of experiments was used to maximize the enzymatic fructose production from crude inulin obtained from Opuntia ficus-indica in a plug-flow mini reactor. The experiments explore temperature (between 45 and 55°C), pH (4.0–5.0), and feed flow (0.1–0.2 ml/min). After verifying the adequacy of the quadratic model for productivity, it was maximized to find the optimal condition. It was at 49.97°C, 4.6 and 0.20 ml/min for the temperature, pH, and flow, respectively. Under the optimal condition, the quadratic model suggested a productivity of 2.456 ± 0.015 mg/h. Three validation experiments confirmed the validity of the model.
Conclusions: The results confirmed the suitability of L. cylindrica as support for the immobilization of inulinase.
References
Rehm FBH, Chen S, Rehm BHA. Bioengineering toward direct production of immobilized enzymes: A paradigm shift in biocatalyst design. Bioengineered 2018;9(1):6-11. https://doi.org/10.1080/21655979.2017.1325040 PMid: 28463573
Singhania RR, Patel AK, Thomas L, et al. Industrial Enzymes. In: Pandey A, Höfer R, Taherzadeh M, et al. editors. Industrial Biorefineries and White Biotechnology 2015:473-97. https://doi.org/10.1016/B978-0-444-63453-5.00015-X
Zhu D, Wu Q, Hua L. Industrial Enzymes. In: Moo-Young M, editor. Comprehensive Biotechnology 2019:1-13. https://doi.org/10.1016/B978-0-444-64046-8.00148-8
Uçkun Kiran E, Trzcinski AP, Ng WJ, et al. Enzyme production from food wastes using a biorefinery concept. Waste Biomass Valorization 2014;5:903-17. https://doi.org/10.1007/s12649-014-9311-x
Ong KL, Kaur G, Pensupa N, et al. Trends in food waste valorization for the production of chemicals, materials and fuels: Case study South and Southeast Asia. Bioresour Technol 2018;248 (part A):100-12. https://doi.org/10.1016/j.biortech.2017.06.076 PMid: 28662903
Ubando AT, Felix CB, Chen WH. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour Technol 2020;299:122585. https://doi.org/10.1016/j.biortech.2019.122585 PMid: 31901305
Ferreira RDG, Azzoni AR, Freitas S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant ?-glucosidase. Biotechnol Biofuels 2018;11:81. https://doi.org/10.1186/s13068-018-1077-0 PMid: 29610578
Honda K. Industrial applications of multistep enzyme reactions. In: Brahmachari G, editor. Biotechnology of Microbial Enzymes. 2017:433-50. https://doi.org/10.1016/B978-0-12-803725-6.00016-9
Di Cosimo R, Mc Auliffe J, Poulose AJ, et al. Industrial use of immobilized enzymes. Chem Soc Rev 2013;42(15):6437-74. https://doi.org/10.1039/c3cs35506c PMid: 23436023
Missau J, Scheid AJ, Foletto EL, et al. Immobilization of commercial inulinase on alginate–chitosan beads. Sustainable Chemical Processes 2014;2:13. https://doi.org/10.1186/2043-7129-2-13
Altunba? C, Uygun M, Uygun DA, et al. Immobilization of inulinase on concanavalin A-attached super macroporous cryogel for production of high-fructose syrup. Appl Biochem Biotechnol 2013;170:1909-21. https://doi.org/10.1007/s12010-013-0322-z PMid: 23780342
Kuhn G de O, Silva MF, Mulinari J, et al. Aspergillus niger inulinase immobilized in polyurethane foam and treated in pressurized LPG: A potential catalyst for enzymatic synthesis of fructooligosaccharides. Biocatal Biotransformation 2016;34(6):291-4. https://doi.org/10.1080/10242422.2016.1247826
Basso A, Serban S. Industrial applications of immobilized enzymes—A review. Molecular Catalysis 2019;479:110607. https://doi.org/10.1016/j.mcat.2019.110607
Chapman J, Ismail AE, Dinu CZ. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018;8(6):238. https://doi.org/10.3390/catal8060238
Azeez MA, Bello OS, Adedeji AO. Traditional and medicinal uses of Luffa cylindrica: A review. Journal of Medicinal Plants Studies 2013;1(5):102–11.
Koseoglu H. Biotemplated Luffa cylindrica for the oil spill clean-up from seawater. Desalination Water Treat 2016;57(57):25591-9. https://doi.org/10.1080/19443994.2016.1152513
Caicedo O, Devia-Ramirez J, Malagón A. Adsorption of common laboratory dyes using natural fibers from Luffa cylindrica. J Chem Educ 2018;95(12):2233-7. https://doi.org/10.1021/acs.jchemed.8b00156
Mahmoud DAR, Refaat HW, Abdel-Fattah AF, et al. Novel application of Luffa cylindrica in production of fructose. Aust J Basic Appl Sci 2011;5(12):2127-37.
Benítez-Cortés I, Pérez-Martínez A, Álvarez-Borroto R, et al. Perspectivas de la producción de inulina a partir de la tuna (Opuntia ficus-indica). Tecnología Química 2015;35:181-92.
Zhu J, Zhang J, Lai Q, et al. Covalent immobilization of lipase onto citric acid-esterified loofah sponge. Bioresources 2013;8:3289–98. https://doi.org/10.15376/biores.8.3.3289-3298
Sumner JB, Graham VA. Dinitrosalicylic acid: A reagent for the estimation of sugar in normal and diabetic urine. Journal of Biological Chemistry 1921;47(1):5-9. https://doi.org/10.1016/S0021-9258(18)86093-8
Abdel-Naby MA, Ismail AMS, Ahmed SA, et al. Production and immobilization of alkaline protease from Bacillus mycoides. Bioresour Technol 1998;64(3):205-10. https://doi.org/10.1016/S0960-8524(97)00160-0
Mashhura Z, Dilrabo F, Sodikjon Z. Dynamics of microorganisms’ producent separation in nutritional environment. International Journal of Innovative Technology and Exploring Engineering 2019;8(9S2):709-11. https://doi.org/10.35940/ijitee.I1147.0789S219

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Electronic Journal of Biotechnology