Abstract
Background: Food and agricultural wastes are produced in huge amounts yearly, putting extra effort into their removal or valorization. One of these wastes is the zucchini heads (ZH) which, in this study, was used as a source to isolate peroxidase using three-phase portioning (TPP).
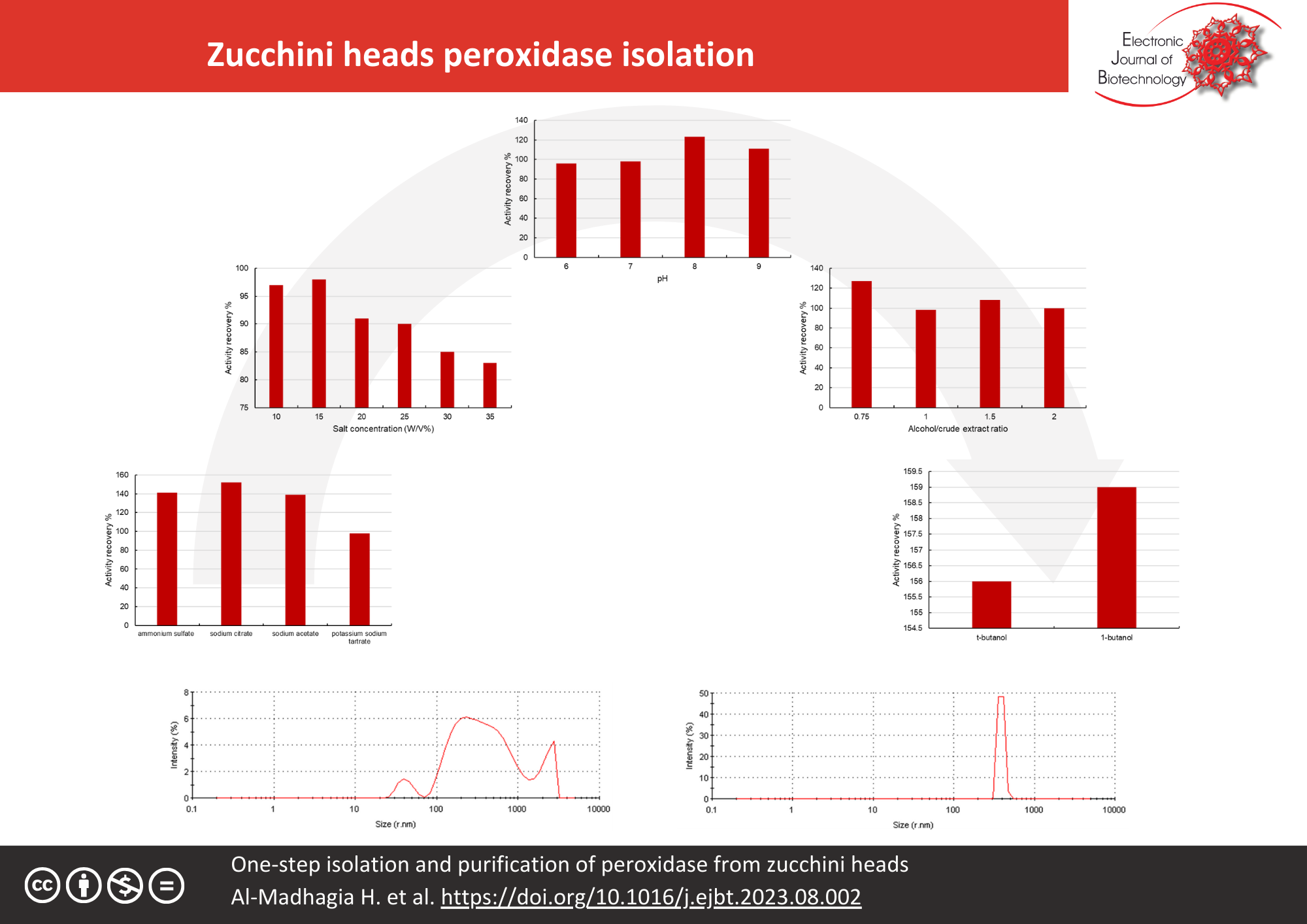
Results: Different parameters of TPP were optimized to ensure obtaining the maximal activity recovery and purity of the enzyme. The purity of the isolated enzyme was performed using the protein homogeneity module of dynamic light scattering. This was followed by the determination of the optimal pH and temperature of the isolated ZH peroxidase. It was found that sodium citrate at a concentration of 15%, pH 8, 1-butanol as the upper alcoholic phase, and an alcohol/crude extract ratio of 0.75:1 were the best conditions for ZH peroxidase isolation. The obtained activity recovery and purification fold were 159% and 10.05, respectively. The isolated ZH peroxidase displayed a high purity as emphasized via dynamic light scattering. The optimum pH and temperature were 8 and 25°C.
Conclusions: The present study was the first to isolate and purify peroxidase from ZH using TPP in one step.
References
de Oliveira FK, Santos LO, Buffon JG. Mechanism of action, sources, and application of peroxidases. Food Research International 2021;143:110266. https://doi.org/10.1016/j.foodres.2021.110266 PMid: 33992367
Passardi F, Longet D, Penel C, et al. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004;65(13):1879-1893. https://doi.org/10.1016/j.phytochem.2004.06.023 PMid: 15279994
Patil PD, Nadar SS, Marghade DT. Photo-enzymatic green synthesis: The potential of combining photo-catalysis and enzymes. In: Inamuddin, Boddula R, Ahamed MI, editors. Advances in green synthesis: Advances in Science, Technology & Innovation. Springer International Publishing; 2021, p 173-189. https://doi.org/10.1007/978-3-030-67884-5_9
Martínez AT, Ruiz-Dueñas FJ, Martínez MJ, et al. Enzymatic delignification of plant cell wall: from nature to mill. Current Opinion in Biotechnology 2009;20(3):348-357. https://doi.org/10.1016/j.copbio.2009.05.002 PMid: 19502047
Gan J, Bilal M, Li X, et al. Peroxidases-based enticing biotechnological platforms for biodegradation and biotransformation of emerging contaminants. Chemosphere 2022;307(Part 3):136035. https://doi.org/10.1016/j.chemosphere.2022.136035 PMid: 35973503
Bilal M, Zdarta J, Jesionowski T, et al. Manganese peroxidases as robust biocatalytic tool — An overview of sources, immobilization, and biotechnological applications. International Journal of Biological Macromolecules 2023;234:123531. https://doi.org/10.1016/j.ijbiomac.2023.123531 PMid: 36754266
Pandey VP, Awasthi M, Singh S, et al. A comprehensive review on function and application of plant peroxidases. Biochemistry & Analytical Biochemistry 2017;6:308. https://doi.org/10.4172/2161-1009.1000308
Basumatary D, Yadav HS, Yadav M. The role of peroxidases in the bioremediation of organic pollutants. The Natural Products Journal 2023;13(1):60-77. https://doi.org/10.2174/2210315512666220410132847
Amor-Gutiérrez O, Costa Rama E, Costa-García A, et al. Paper-based maskless enzymatic sensor for glucose determination combining ink and wire electrodes. Biosensors Bioelectronics 2017;93:40-45. https://doi.org/10.1016/j.bios.2016.11.008 PMid: 27856164
Rastogi L, Dash K, Sashidhar RB. Selective and sensitive detection of cholesterol using intrinsic peroxidase-like activity of biogenic palladium nanoparticles. Current Research in Biotechnology 2021;3:42-48. https://doi.org/10.1016/j.crbiot.2021.02.001
Singh G, Kushwaha A, Sharma M. Intriguing peroxidase-mimic for H2O2 and glucose sensing: A synergistic Ce2(MoO4)3/rGO nanocomposites. Journal of Alloys and Compounds 2020;825:154134. https://doi.org/10.1016/j.jallcom.2020.154134
Veitch NC. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004;65(3):249-259. https://doi.org/10.1016/j.phytochem.2003.10.022 PMid: 14751298
Mohamed SA, Abulnaja KO, Ads AS, et al. Characterisation of an anionic peroxidase from horseradish cv. Balady. Food Chemistry 2011;128(3):725-730. https://doi.org/10.1016/j.foodchem.2011.03.096
Krainer FW, Glieder A. An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Applied Microbiology and Biotechnology 2015;99:1611-1625. https://doi.org/10.1007/s00253-014-6346-7 PMid: 25575885
Almulaiky YQ. Peroxidase from Coleus forskohlii: Purification and Biochemical Characterization. IJN 2020;5(1):9-20. https://doi.org/10.14302/issn.2379-7835.ijn-19-3139
Zeyadi M, Almulaiky YQ. A novel peroxidase from Ziziphus jujuba fruit: Purification, thermodynamics and biochemical characterization properties. Sci Rep 2020;10:8007. https://doi.org/10.1038/s41598-020-64599-9 PMid: 32409642
Almulaiky YQ, Al-Harbi SA. A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. International Journal of Biological Macromolecules 2019;133:767-174. https://doi.org/10.1016/j.ijbiomac.2019.04.119 PMid: 31004641
Yan J-K, Wang Y-Y, Qiu W-Y, et al. Three-phase partitioning as an elegant and versatile platform applied to nonchromatographic bioseparation processes. Critical Reviews in Food Science and Nutrition 2018;58(14):2416-2431. https://doi.org/10.1080/10408398.2017.1327418 PMid: 28609145
Chew KW, Ling TC, Show PL. Recent developments and applications of three-phase partitioning for the recovery of proteins. Separation & Purification Reviews 2019;48(1):52-64. https://doi.org/10.1080/15422119.2018.1427596
Narayan AV, Madhusudhan MC, Raghavarao KSMS. Extraction and purification of Ipomoea peroxidase employing three-phase partitioning. Applied Biochemistry and Biotechnology 2008;151:263-272. https://doi.org/10.1007/s12010-008-8185-4 PMid: 18369532
Yuzugullu Karakus Y, Acemi A, I??k S, et al. Purification of peroxidase from Amsonia orientalis by three-phase partitioning and its biochemical characterization. Separation Science and Technology 2018;53(5):756-766. https://doi.org/10.1080/01496395.2017.1405990
Markwell MAK, Haas SM, Bieber LL, et al. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Analytical Biochemistry 1978;87(1):206-210). https://doi.org/10.1016/0003-2697(78)90586-9 PMid: 98070
Stetefeld J, McKenna SA, Patel TR. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophysical Reviews 2016;8:409-427. https://doi.org/10.1007/s12551-016-0218-6 PMid: 28510011
Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresources and Bioprocessing 2018;5:1. https://doi.org/10.1186/s40643-017-0187-z
Yusree FIFM, Peter AP, Mohd Nor MZ, et al. Latest advances in protein-recovery technologies from agricultural waste. Foods 2021;10(11):2748. https://doi.org/10.3390/foods10112748 PMid: 34829028
Vidhate GS, Singhal RS. Extraction of cocoa butter alternative from kokum (Garcinia indica) kernel by three phase partitioning. Journal of Food Engineering 2013;117(4):464-466. https://doi.org/10.1016/j.jfoodeng.2012.10.051
Ketnawa S, Rungraeng N, Rawdkuen S. Phase partitioning for enzyme separation: An overview and recent applications. International Food Research Journal 2017;24(1):1-24.
Kublicki M, Koszelewski D, Brodzka A, et al. Wheat germ lipase: Isolation, purification and applications. Critical Reviews in Biotechnology 2022;42(2):184-200. https://doi.org/10.1080/07388551.2021.1939259 PMid: 34266327
Gul A, Khan S, Arain H, et al. Three-phase partitioning as an efficient one-step method for the extraction and purification of bromelain from pineapple crown waste. Journal of Food Processing and Preservation 2022;46(11):e16973. https://doi.org/10.1111/jfpp.16973
Jain J. Review on isolation and purification of papain enzyme from papaya fruit. International Journal of Engineering Applied Sciences and Technology 2020;5(6):193-197. https://doi.org/10.33564/IJEAST.2020.v05i06.028
Eyssen LE, Goldring JPD, Coetzer THT. Three-phase partitioning (TPP) of proteases from parasites, plants, tissue and bacteria for enhanced activity. In: Gupta M, Roy I, editors. Three Phase Partitioning, Elsevier; 2021, p 133-154. https://doi.org/10.1016/B978-0-12-824418-0.00008-4
Gagaoua M, Hafid K. Three phase partitioning system, an emerging non-chromatographic tool for proteolytic enzymes recovery and purification. Biosensors Journal 2016;5(1):100134.
Vetal MD, Rathod VK. Three phase partitioning a novel technique for purification of peroxidase from orange peels (Citrus sinenses). Food and Bioproducts Processing 2015;94:284-289. https://doi.org/10.1016/j.fbp.2014.03.007
Vetal MD, Rathod VK. Ultrasound assisted three phase partitioning of peroxidase from waste orange peels. Green Processing and Synthesis 2016;5(2):205-212 https://doi.org/10.1515/gps-2015-0116
Panadare DC, Rathod VK. Extraction of peroxidase from bitter gourd (Momordica charantia) by three phase partitioning with dimethyl carbonate (DMC) as organic phase. Process Biochemistry 2017;61:195-201. https://doi.org/10.1016/j.procbio.2017.06.028

This work is licensed under a Creative Commons Attribution 4.0 International License.