Abstract
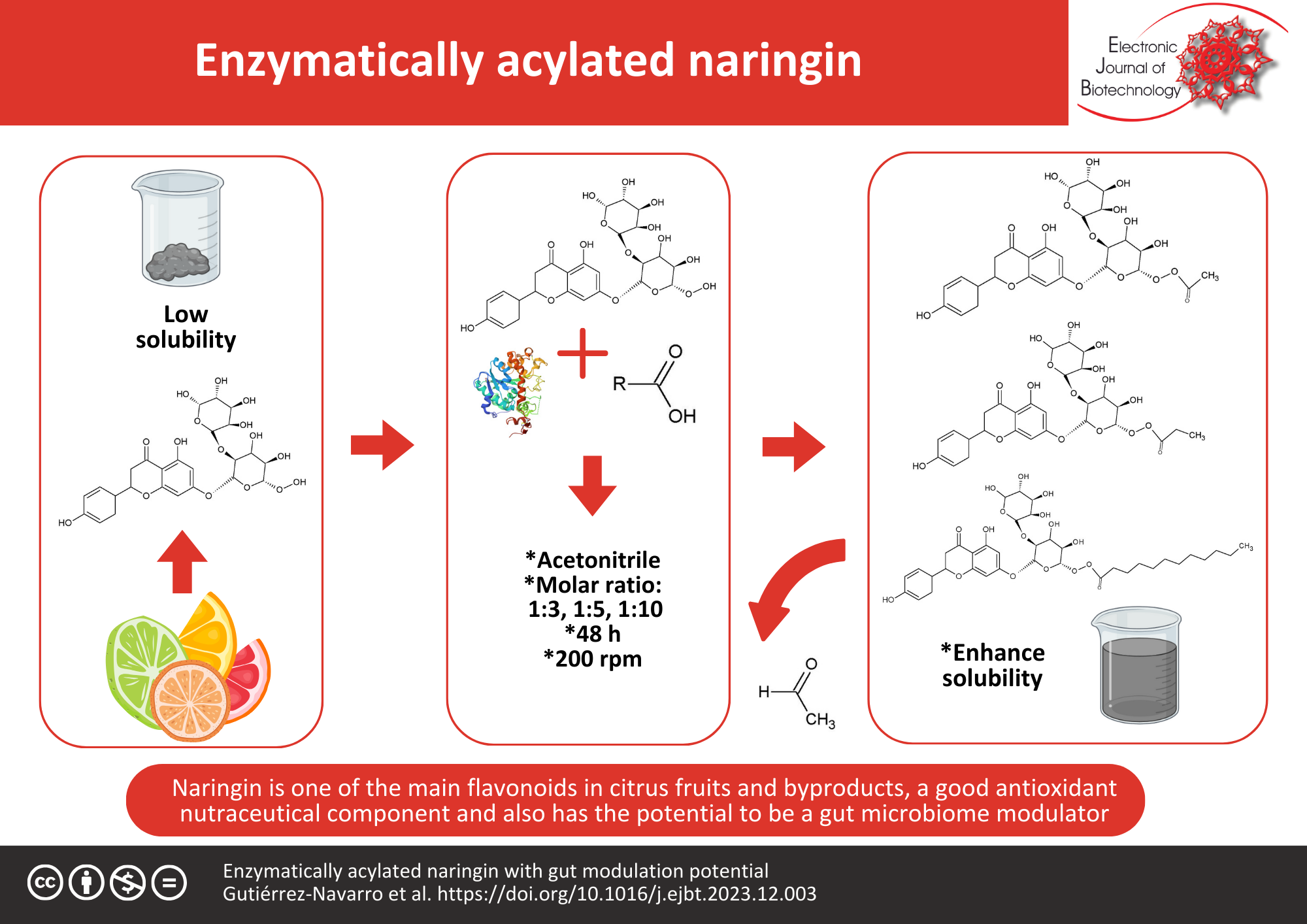
Background: Naringin is one of the main flavonoids in citrus fruits and byproducts. This flavanone has been shown to be a good antioxidant nutraceutical component, and it also has potential as a gut microbiome modulator, although its applications in final formulations represent a challenge due to its low solubility, both in water and in organic solvents. This work addresses this problem by functionalizing naringin through enzymatic acylation.
Results: The enzymatic acylation catalyzed by the lipase Novozym® 435 and using acyl donors of different chain lengths, acetate (C2), propionate (C3), and laurate (C12), yielded in conversions of 95% at 24 h and 100% at 48 h, generating a monoacylated product. Both the aqueous and solvent solubility of acylated naringin products were improved while maintaining or even increasing their antioxidant activity.
Conclusions: This acylation process significantly enhanced both the water and solvent solubility of the acylated naringin products while preserving or even enhancing their antioxidant activity. In addition to the gut-modulating properties of flavonoids, acylating them with short- and medium-chain fatty acids could enhance their potential applications in the emerging field of research dedicated to understanding and modulating gut health.
References
Barreca D, Mandalari G, Calderaro A, et al. Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants 2020;9(3):288. https://doi.org/10.3390/plants9030288 PMid: 32110931
Coello F, Peraza-Marrero M, Pinto-Catari I. Flavonoides: Micronutrientes con amplia actividad biológica. Revista de la Facultad de Medicina, Universidad Nacional de Colombia 2021;44:108-126.
Tripoli E, Guardia ML, Giammanco S, et al. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem 2007;104(2):466-479. https://doi.org/10.1016/j.foodchem.2006.11.054
Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem 2019;299:125124. https://doi.org/10.1016/j.foodchem.2019.125124 PMid: 31288163
Wojnar W, Zych M, Kaczmarczyk-Sedlak I. Antioxidative effect of flavonoid naringenin in the lenses of type 1 diabetic rats. Biomed Pharmacother 2018;108(2018):974-984. https://doi.org/10.1016/j.biopha.2018.09.092 PMid:30372909
Ali AM, Gabbar MA, Abdel-Twab SM, et al. Antidiabetic potency, antioxidant effects, and mode of actions of Citrus reticulata fruit peel hydroethanolic extract, hesperidin, and quercetin in nicotinamide/streptozotocin-induced Wistar diabetic rats. Oxid Med Cell Longev 2020;2020:1730492. https://doi.org/10.1155/2020/1730492 PMid: 7327566
Jucá MM, Cysne Filho FMS, de Almeida JC, et al. Flavonoids: Biological activities and therapeutic potential. Nat Prod Res 2020;34(5):692-705. https://doi.org/10.1080/14786419.2018.1493588 PMid: 30445839
Padilla de la Rosa JD, Ruiz-Palomino P, Arriola-Guevara E, et al. A green process for the extraction and purification of hesperidin from Mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the Citrus genus. Processes 2018;6(12):266. https://doi.org/10.3390/pr6120266
Rodríguez-Daza MC, Pulido-Mateos EC, Lupien-Meilleur J, et al. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front Nutr 2021;8(2021):689456. https://doi.org/10.3389/fnut.2021.689456 PMid: 34268328
Sost MM, Ahles S, Verhoeven J, et al. A citrus fruit extract high in polyphenols beneficially modulates the gut microbiota of healthy human volunteers in a validated in vitro model of the colon. Nutrients 2021;13(11):3915. https://doi.org/10.3390/nu13113915 PMid: 34836169
Sakao K, Hou D-X. Effects and mechanisms of acylated flavonoid on cancer chemopreventive activity. Curr Pharmacol Rep 2020;6(5):286-305. https://doi.org/10.1007/s40495-020-00233-6
Choi G-Y, Kim H-B, Hwang E-S, et al. Naringin enhances long-term potentiation and recovers learning and memory deficits of amyloid-beta induced Alzheimer’s disease-like behavioral rat model. Neurotoxicology 2023;95(2023):35-45. https://doi.org/10.1016/j.neuro.2022.12.007 PMid: 36549596
Long J-Y, Chen J-M, Liao Y-J, et al. Naringin provides neuroprotection in CCL2-induced cognition impairment by attenuating neuronal apoptosis in the hippocampus. Behav Brain Funct 2020;16(1):4. https://doi.org/10.1186/s12993-020-00166-6 PMid: 32103758
Ahmed S, Khan H, Aschner M, et al. Therapeutic potential of naringin in neurological disorders. Food Chem Toxicol 2019;132(2019):110646. https://doi.org/10.1016/j.fct.2019.110646 PMid: 20811644
Goldwasser J, Cohen PY, Yang E, et al. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPAR?, PPAR? and LXR?. PLOS ONE 2010;5(8):e12399. https://doi.org/10.1371/journal.pone.0012399 PMid: 20811644
Shi Y, Tan YAN, Mao S, et al. Naringenin inhibits allergen?induced airway remodeling in a murine model of asthma. Mol Med Rep 2014;9(4):1204-1208. https://doi.org/10.3892/mmr.2014.1940 PMid: 24534822
Wang W, Mao J, Chen Y, et al. Naringin promotes osteogenesis and ameliorates osteoporosis development by targeting JAK2/STAT3 signaling. Clin Exp Pharmacol Physiol 2021;49(1):113-121. https://doi.org/10.1111/1440-1681.13591 PMid: 34525226
Cai L, Wu H, Tu C, et al. Naringin inhibits ovarian tumor growth by promoting apoptosis: An in vivo study. Oncol Lett 2018;16(1):59-64. https://doi.org/10.3892/ol.2018.8611 PMid: 8910296
Passamonti S, Terdoslavich M, Franca R, et al. Bioavailability of flavonoids: a review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr Drug Metab 2009;10(4):369-394. https://doi.org/10.2174/138920009788498950 PMid: 19519345
You HJ, Ahn HJ, Ji GE. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J Agric Food Chem 2010;58(20):10886-10892. https://doi.org/10.1021/jf102871g PMid: 20886886
Pereira-Caro G, Polyviou T, Ludwig IA, et al. Bioavailability of orange juice (poly)phenols: the impact of short-term cessation of training by male endurance athletes. Am J Clin Nutr 2017;106(3):791-800. https://doi.org/10.3945/ajcn.116.149898 PMid: 28747329
Cao R, Wu X, Guo H, et al. Naringin exhibited therapeutic effects against DSS-induced mice ulcerative colitis in intestinal barrier–dependent manner. Molecules 2021;26:6604. https://doi.org/10.3390/molecules26216604 PMid: 34771012
Fidélix M, Milenkovic D, Sivieri K, et al. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food & Funct 2020;11(2):1599-1610. https://doi.org/10.1039/C9FO02623A PMid: 32016250
Negatu DA, Gengenbacher M, Dartois V, et al. Indole propionic acid, an unusual antibiotic produced by the gut microbiota, with anti-inflammatory and antioxidant properties. Front Microbiol 2020;11(2020):575586. https://doi.org/10.3389/fmicb.2020.575586 PMid:33193190
Gao X, Lin S-H, Ren F, et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun 2016;7(1):11960. https://doi.org/10.1038/ncomms11960 PMid: 27357947
Hernandez MAG, Canfora EE, Jocken JWE, et al. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019;11(8):1943. https://doi.org/10.3390/nu11081943 PMid: 31426593
He J, Zhang P, Shen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci 2020;21(17):6356. https://doi.org/10.3390/ijms21176356 PMid: 32887215
Sheela DL, Narayanankutty A, Nazeem PA, et al. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum Exp Toxicol 2019;38(7):753-761. https://doi.org/10.1177/0960327119839185 PMid: 30942101
Nitbani FO, Tjitda PJP, Nitti F, et al. Antimicrobial properties of lauric acid and monolaurin in virgin coconut oil: A review. ChemBioEng Rev 2022;9(5):442-461. https://doi.org/10.1002/cben.202100050
Reyes-Reyes AL, Valero Barranco F, Sandoval G. Recent advances in lipases and their applications in the food and nutraceutical industry. Catalysts 2022;12(9):960. https://doi.org/10.3390/catal12090960
Chebil L, Humeau C, Falcimaigne A, et al. Enzymatic acylation of flavonoids. Process Biochem 2006;41(11):2237-2251. https://doi.org/10.1016/j.procbio.2006.05.027
Reyes-Reyes AL, Valero F, Sandoval G. Cloning, protein expression and biochemical characterization of Carica papaya esterase. Electron J Biotechnol 2023;61(2023):61-68. https://doi.org/https://doi.org/10.1016/j.ejbt.2022.11.004
Milisavljevi? A, Stojanovi? M, Carevi? M, et al. Lipase-catalyzed esterification of phloridzin: acyl donor effect on enzymatic affinity and antioxidant properties of esters. Ind Eng Chem Res 2014;53(43):16644-16651. https://doi.org/10.1021/ie5027259
Loucif K, Benabdallah H, Benchikh F, et al. Total phenolic contents, DPPH radical scavenging and ?-carotene bleaching activities of aqueous extract from Ammoides atlantica. J Drug Delivery & Ther 2020;10(3-S):196-198. https://doi.org/10.22270/jddt.v10i3-s.4151
Nogales-Delgado S, Encinar JM, González JF. Safflower biodiesel: Improvement of its oxidative stability by using BHA and TBHQ. Energies 2019;12(10):1940. https://doi.org/10.3390/en12101940
Lu P, Chen Y, Tan M et al. Chemical profiling by LC–MS/MS and HPLC fingerprint combined with chemometrics and simultaneous determination of 16 characteristic ingredients for the quality consistency evaluation of Shaoyao-Gancao Decoction. Biomed Chromatogr 2019;33(2):e4401. https://doi.org/10.1002/bmc.4401 PMid: 30277266
Doerr M, Romero A, Daza MC. Effect of the acyl-group length on the chemoselectivity of the lipase-catalyzed acylation of propranolol-a computational study. J Mol Model 2021;27(7):198. https://doi.org/10.1007/s00894-021-04808-y PMid: 34115202
Sandoval G, Quintana PG, Baldessari A, et al. Lipase-catalyzed preparation of mono- and diesters of ferulic acid. Biocatal Biotransform 2015;33(2):89-97. https://doi.org/10.3109/10242422.2015.1060228
Sandoval G, Condoret JS, Monsan P, et al. Esterification by immobilized lipase in solvent-free media: Kinetic and thermodynamic arguments. Biotechnol Bioeng 2002;78(3):313-320. https://doi.org/10.1002/bit.10224 PMid: 11920447
González-Alfonso JL, Míguez N, Padilla JD, et al. Optimization of regioselective ?-glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. Molecules 2018;23(11):2885. https://doi.org/10.3390/molecules23112885 PMid: 30400664
Silvestrini L, Cianci M. Principles of lipid-enzyme interactions in the limbus region of the catalytic site of Candida antarctica Lipase B. Int J Biol Macromol 2020;158(2020):358-363. https://doi.org/10.1016/j.ijbiomac.2020.04.061 PMid: 32380114
Milivojevi? A, ?orovi? M, Carevi? M, et al. Highly efficient enzymatic acetylation of flavonoids: Development of solvent-free process and kinetic evaluation. Biochem Eng J 2017;128(2017):106-115. https://doi.org/10.1016/j.bej.2017.09.018
Yang W, Kortesniemi M, Ma X, et al. Enzymatic acylation of blackcurrant (Ribes nigrum) anthocyanins and evaluation of lipophilic properties and antioxidant capacity of derivatives. Food Chem 2019;281:189-196. https://doi.org/10.1016/j.foodchem.2018.12.111 PMid: 30658747
Céliz G, Daz M. Biocatalytic preparation of alkyl esters of citrus flavanone glucoside prunin in organic media. Process Biochem 2011;46(1):94-100. https://doi.org/10.1016/j.procbio.2010.07.022
Mellou F, Lazari D, Skaltsa H, et al. Biocatalytic preparation of acylated derivatives of flavonoid glycosides enhances their antioxidant and antimicrobial activity. J Biotechnol 2005;116(3):295-304. https://doi.org/https://doi.org/10.1016/j.jbiotec.2004.12.002 PMid: 15707690
Sun CQ, Johnson KD, Wong H, et al. Biotransformation of flavonoid conjugates with fatty acids and evaluations of their functionalities. Front Pharmacol 2017;8(2017):759. https://doi.org/10.3389/fphar.2017.00759 PMid: 29163154
Guo H, Yu J, Lei B, et al. T enzymatic esterification of naringin and the properties of naringin esterified derivatization. Ind Crops Prod 2022;176(2022):114372. https://doi.org/10.1016/j.indcrop.2021.114372
Qian J, Gou L, Chen Y, et al. Enzymatic acylation of flavone isolated from extractive of bamboo leaves with oleic acid and antioxidant activity of acylated product. Eng Life Sci 2019;19(1):66-72. https://doi.org/10.1002/elsc.201800096 PMid: 32624957
Jasi?ska K, Fabiszewska A, Bia?ecka-Florja?czyk E, et al. Mini-review on the enzymatic lipophilization of phenolics present in plant extracts with the special emphasis on anthocyanins. Antioxidants 2022;11(8):1528. https://doi.org/10.3390/antiox11081528 PMid: 36009246
Li HM, Xu TT, Peng QX, et al. Enzymatic acylation of rutin with benzoic acid ester and lipophilic, antiradical, and antiproliferative properties of the acylated derivatives. J Food Sci 2021;86(5):1714-1725. https://doi.org/10.1111/1750-3841.15703 PMid: 33844282
Padilla-Camberos E, Arrizon J, Sandoval G. Effect of agave fructan bioconjugates on metabolic syndrome parameters in a murine model. Pharmaceuticals 2023;16(3):412. https://doi.org/10.3390/ph16030412 PMid: 36986511
Lippolis T, Cofano M, Caponio GR, et al. Bioaccessibility and bioavailability of diet polyphenols and their modulation of gut microbiota. Int J Mol Sci 2023;24(4):3813. https://doi.org/10.3390/ijms24043813 PMid: 36835225
Pei R, Liu X, Bolling B. Flavonoids and gut health. Curr Opin Biotechnol 2020;61(2020):153-159. https://doi.org/10.1016/j.copbio.2019.12.018 PMid: 31954357

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Electronic Journal of Biotechnology