Abstract
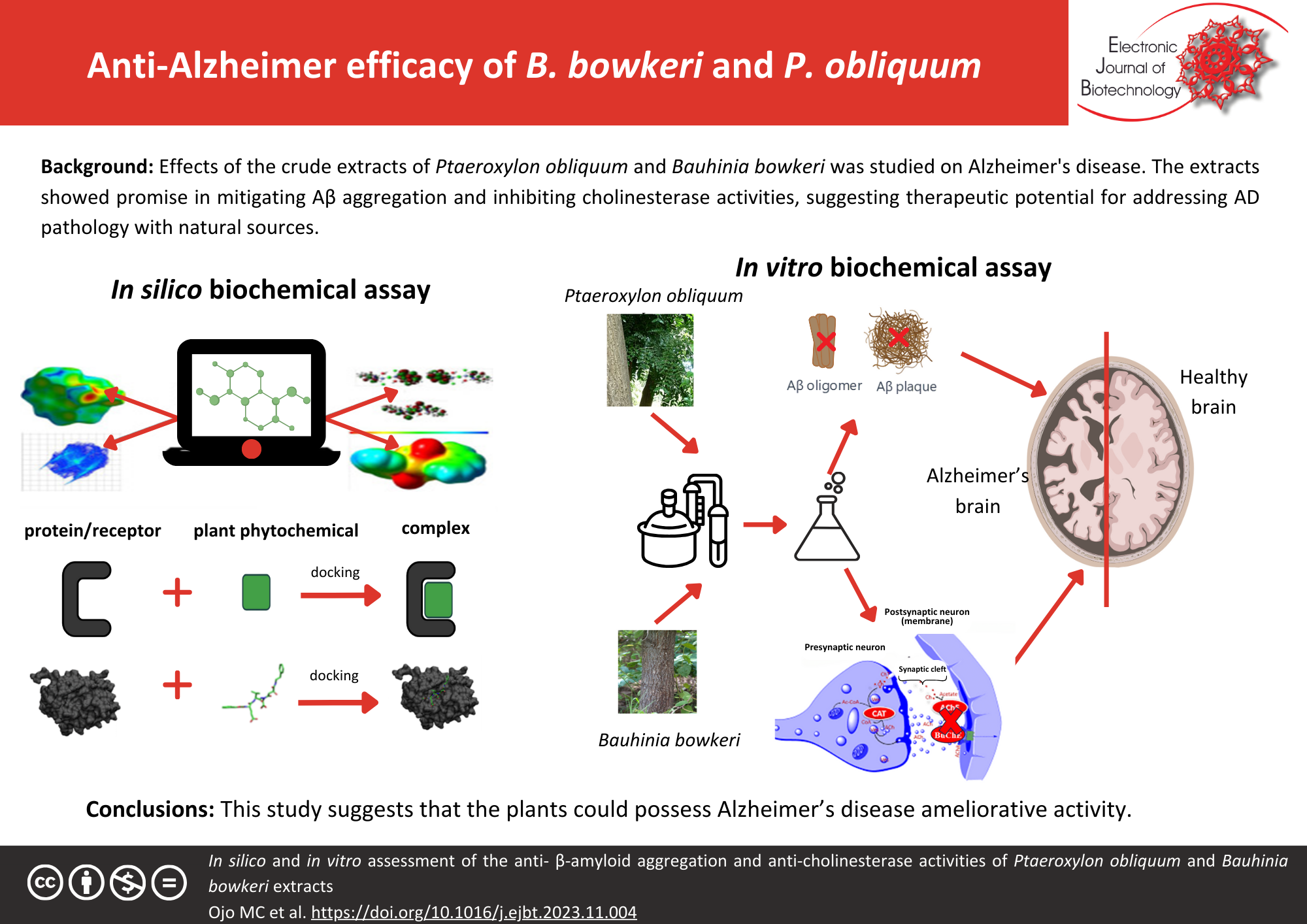
Background: Alzheimer’s disease (AD) is the most common form of dementia and dementia constitutes the fifth leading cause of mortality across the globe. Available treatment modalities and drugs have abysmally failed to curtail AD. This study evaluated the mitigation of Aβ aggregation and anti-cholinesterase activities with the crude extracts of Ptaeroxylon obliquum and Bauhinia bowkeri. Computational studies of the most abundant phytochemicals from the crude extracts of both plants with proteins were investigated. The phytochemical composition of the different crude extracts (hexane, DCM, and ethanol) of the plants were analyzed with FTIR and GC-MS. The inhibitory potential of the extracts on BACE-1 and cholinesterase activities was determined with both computational molecular docking studies and in vitro enzyme assays. Their anti-aggregation properties were confirmed with Thioflavin-T assay and TEM.
Results: The in silico studies revealed that though thunbergol and cyclotetradecatriene (the major constituents of the extracts) inhibited all the proteins, the latter exhibited the best inhibitory potential. The in vitro results showed that while the dichloromethane (DCM) extract of P. obliquum had the highest butyrylcholinesterase (BuChE) inhibitory activity (1.77 µg/ml), the hexane and ethanol extract of B. bowkeri exhibited the highest β-site amyloid precursor cleaving enzymes-1 (BACE-1) (30.4 µg/ml) and acetylcholinesterase (AChE) (58.11 µg/ml) inhibitory efficacy, respectively. The ethanol extract (160 μg/ml) of B. bowkeri had the most efficacious anti-aggregation activity.
Conclusions: This study suggests that the plants could possess neuroprotective effects and could also be sources of anti-AD novel drugs.
References
Zhu Z, Lin Y, Li X, et al. Shared genetic architecture between metabolic traits and Alzheimer’s disease: a large-scale genome-wide cross-trait analysis. Human Genetics 2019;138:271-85. https://doi.org/10.1007/s00439-019-01988-9 PMid: 30805717
Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Scientific Reports. 2017;7(1):2426. https://doi.org/10.1038/s41598-017-02587-2 PMid: 28546539
Guerreiro R, Hardy J. Genetics of Alzheimer’s disease. Neurotherapeutics 2014;11:732-7. https://doi.org/10.1007/s13311-014-0295-9 PMid: 25113539
Agis-Torres A, Sollhuber M, Fernandez M, et al. Multi-target-directed ligands and other therapeutic strategies in the search of a real solution for Alzheimer’s disease. Current Neuropharmacology 2014;12(1):2-36. https://doi.org/10.2174/1570159X113116660047 PMid: 24533013
Zhao Y, Gong CX. From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration. Cellular and Molecular Neurobiology 2015;35:101-10. https://doi.org/10.1007/s10571-014-0127-9 PMid: 25352419
Ayton S, Lei P, Bush AI. Metallostasis in Alzheimer's disease. Free Radical Biology and Medicine. 2013;62:76-89. https://doi.org/10.1016/j.freeradbiomed.2012.10.558 PMid: 23142767
Tampi RR. Paired associative stimulation (PAS) and Alzheimer’s disease (AD). International Psychogeriatrics 2023;35(3):123-5. https://doi.org/10.1017/S1041610222000813 PMid: 36098440
2023 Alzheimer’s disease facts and figures. Alzheimers & Dementia 2023;19(4):1598–1695. https://doi.org/10.1002/alz.13016 PMid: 36918389
Viayna E, Coquelle N, Cieslikiewicz-Bouet M, et al. Discovery of a potent dual inhibitor of acetylcholinesterase and butyrylcholinesterase with antioxidant activity that alleviates Alzheimer-like pathology in old APP/PS1 mice. Journal of Medicinal Chemistry 2020;64(1):812-39. https://doi.org/10.1021/acs.jmedchem.0c01775 PMid: 33356266
Ajala A, Uzairu A, Shallangwa GA, et al. Structure-based drug design of novel piperazine containing hydrazone derivatives as potent Alzheimer inhibitors: molecular docking and drug kinetics evaluation. Brain Disorders 2022;7:100041. https://doi.org/10.1016/j.dscb.2022.100041
Abraha A, Ghoshal N, Gamblin TC, et al. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. Journal of Cell Science 2000;113(21):3737-45. https://doi.org/10.1242/jcs.113.21.3737 PMid: 11034902
Hong L, Turner III RT, Koelsch G, et al. Memapsin 2 (?-secretase) as a therapeutic target. Biochemical Society Transactions 2002;30(4):530-4. https://doi.org/10.1042/bst0300530 PMid: 12196130
Amani M, Shokouhi G, Salari AA. Minocycline prevents the development of depression-like behavior and hippocampal inflammation in a rat model of Alzheimer's disease. Psychopharmacology 2019;236(4):1281-1292. https://doi.org/10.1007/s00213-018-5137-8 PMid: 30515523
Thakur A, Chun YS, October N, et al. Potential of South African medicinal plants targeting the reduction of A?42 protein as a treatment of Alzheimer's disease. Journal of Ethnopharmacology 2019;231:363-73. https://doi.org/10.1016/j.jep.2018.11.034 PMid: 30496841
Hook V, Schechter I, Demuth HU, et al. Alternative pathways for production of ?-amyloid peptides of Alzheimer's disease. Biological Chemistry 2008;389(8):993-1006. https://doi.org/10.1515/BC.2008.124 PMid: 18979625
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297(5580):353-6. https://doi.org/10.1126/science.1072994 PMid: 12130773
Westmark CJ. What’s hAPPening at synapses? The role of amyloid ?-protein precursor and ?-amyloid in neurological disorders. Molecular psychiatry. 2013;18(4):425-34. https://doi.org/10.1038/mp.2012.122 PMid: 22925831
Ogunruku OO, Oboh G, Passamonti S, et al. Capsicum annuum var. grossum (Bell pepper) inhibits ?-secretase activity and ?-amyloid1–40 aggregation. Journal of Medicinal Food 2017;20(2):124-30. https://doi.org/10.1089/jmf.2016.0077 PMid: 28098506
Sobhani R, Pal AK, Bhattacharjee A, et al. Screening indigenous medicinal plants of northeast India for their anti-Alzheimer’s properties. Pharmacognosy Journal 2017;9(1):46-54. https://doi.org/10.5530/pj.2017.1.9
Coimbra JR, Marques DF, Baptista SJ, et al. Highlights in BACE1 inhibitors for Alzheimer's disease treatment. Frontiers in Chemistry 2018;6:178. https://doi.org/10.3389/fchem.2018.00178 PMid: 29881722
Adeola HA, Sabiu S, Aruleba RT, et al. Phytodentistry in Africa: prospects for head and neck cancers. Clinical Phytoscience 2021;7(1):17. https://doi.org/10.1186/s40816-021-00254-8
Stojanoski N. Development of health culture in Veles and its region from the past to the end of the 20th century. Veles: Society of science and art 1999:13-34.
Kelly K. The History of Medicine: Early Civilizations, Prehistoric Times to 500 CE. Ferguson Publishing Company; 2009, 174 p
Ahmed AS, Elgorashi EE, Moodley N, et al. The antimicrobial, antioxidative, anti-inflammatory activity and cytotoxicity of different fractions of four South African Bauhinia species used traditionally to treat diarrhoea. Journal of Ethnopharmacology 2012;143(3):826-839. https://doi.org/10.1016/j.jep.2012.08.004 PMid: 22917809
Ndawonde BG, Zobolo AM, Dlamini ET, et al. A survey of plants sold by traders at Zululand muthi markets, with a view to selecting popular plant species for propagation in communal gardens. African Journal of Range and Forage Science 2007;24(2):103-7. https://doi.org/10.2989/AJRFS.2007.24.2.7.161
Baloyi IT, Cosa S, Combrinck S, et al. Anti-quorum sensing and antimicrobial activities of South African medicinal plants against uropathogens. South African Journal of Botany 2019;122:484-91. https://doi.org/10.1016/j.sajb.2019.01.010
Van Jaarsveld E. Veld gardening in South Africa. Veld & Flora. 1996;82(2):52-6.
Khare P, Kishore K, Sharma DK. Historical aspects, medicinal uses, phytochemistry and pharmacological review of Bauhinia variegate. Asian J Pharm Pharmacol 2018;4(5):546-62. https://doi.org/10.31024/ajpp.2018.4.5.3
Ramadwa TE, McGaw LJ, Adamu M, et al. Anthelmintic, antimycobacterial, antifungal, larvicidal and cytotoxic activities of acetone leaf extracts, fractions and isolated compounds from Ptaeroxylon obliquum (Rutaceae). Journal of Ethnopharmacology 2021;280:114365. https://doi.org/10.1016/j.jep.2021.114365 PMid: 34175445
Van Wyk C, Botha FS, Vleggaar R, et al. Obliquumol, a novel antifungal and a potential scaffold lead compound, isolated from the leaves of Ptaeroxylon obliquum (sneezewood) for treatment of Candida albicans infections. Suid-Afrikaanse Tydskrif vir Natuurwetenskap en Tegnologie. 2018;37(1):1-5.
Mwinga JL, Makhaga NS, Aremu AO, et al. Botanicals used for cosmetic purposes by Xhosa women in the Eastern Cape, South Africa. South African Journal of Botany 2019;126:4-10. https://doi.org/10.1016/j.sajb.2019.03.038
Gorun V, Proinov I, B?ltescu V, et al. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Analytical Biochemistry 1978;86(1):324-6. https://doi.org/10.1016/0003-2697(78)90350-0 PMid: 655393
Olasehinde TA, Odjadjare EC, Mabinya LV, et l. Chlorella sorokiniana and Chlorella minutissima exhibit antioxidant potentials, inhibit cholinesterases and modulate disaggregation of ?-amyloid fibrils. Electronic Journal of Biotechnology. 2019;40:1-9. https://doi.org/10.1016/j.ejbt.2019.03.008
Nilsson P, Loganathan K, Sekiguchi M, et al. A? secretion and plaque formation depend on autophagy. Cell Reports 2013;5(1):61-9. https://doi.org/10.1016/j.celrep.2013.08.042 PMid: 24095740
Oset-Gasque MJ, Marco-Contelles J. Alzheimer’s disease, the “one-molecule, one-target” paradigm, and the multitarget directed ligand approach. ACS Chemical Neuroscience 2018;9(3):401-3. https://doi.org/10.1021/acschemneuro.8b00069 PMid: 29465220
Kumari A, Sharma R, Shrivastava N, et al. Bleomycin modulates amyloid aggregation in ?-amyloid and hIAPP. RSC Advances 2020;10(43):25929-46. https://doi.org/10.1039/D0RA04949B PMid: 35518630
Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nature Reviews Drug Discovery 2004;3(8):673-83. https://doi.org/10.1038/nrd1468 PMid: 15286734
Bibi N, Rizvi S, Batool A, et al. Inhibitory mechanism of an anticancer drug, bexarotene against amyloid ? peptide aggregation: repurposing via neuroinformatics approach. Current Pharmaceutical Design 2019;25(27):2989-95. https://doi.org/10.2174/1381612825666190801123235 PMid: 31368868
Breckenridge A, Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nature Reviews Drug Discovery 2019;18(1):1-2. https://doi.org/10.1038/nrd.2018.92 PMid: 29880920
Oliveira RC, Bandeira PN, Lemos TL, et al. In silico and in vitro evaluation of efflux pumps inhibition of ?, ?-amyrin. Journal of Biomolecular Structure and Dynamics. 2022;40(23):12785-99. https://doi.org/10.1080/07391102.2021.1976277 PMid: 34528866
Samreen, Qais FA, Ahmad I. In silico screening and in vitro validation of phytocompounds as multidrug efflux pump inhibitor against E. coli. Journal of Biomolecular Structure and Dynamics. 2023;41(6):2189-201. https://doi.org/10.1080/07391102.2022.2029564 PMid: 35067192
Panzella L, Eidenberger T, Napolitano A. Anti-amyloid aggregation activity of black sesame pigment: toward a novel Alzheimer’s disease preventive agent. Molecules 2018;23(3):676. https://doi.org/10.3390/molecules23030676 PMid: 29547584
Meden A, Knez D, Juki? M, et al. Tryptophan-derived butyrylcholinesterase inhibitors as promising leads against Alzheimer's disease. Chemical Communications 2019;55(26):3765-8. https://doi.org/10.1039/C9CC01330J PMid: 30864579
Pajk S, Knez D, Košak U, et al. Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes. Journal of Enzyme Inhibition and Medicinal Chemistry 2020;35(1):498-505. https://doi.org/10.1080/14756366.2019.1710502 PMid: 31914836
Aparna V, Dileep KV, Mandal PK, et al. Anti?inflammatory property of n?hexadecanoic acid: structural evidence and kinetic assessment. Chemical Biology & Drug Design 2012;80(3):434-9. https://doi.org/10.1111/j.1747-0285.2012.01418.x PMid: 22642495
Servi H, Sen A, Dogan A. Chemical composition and biological activities of endemic Tripleurospermum conoclinium (Boiss. & Balansa) Hayek essential oils. Flavour and Fragrance Journal 2020;35(6):713-21. https://doi.org/10.1002/ffj.3610
do Nascimento KF, Moreira FM, Santos JA, et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. Journal of Ethnopharmacology 2018;210:351-8. https://doi.org/10.1016/j.jep.2017.08.030 PMid: 28844678
Sufriadi E, Meilina H, Munawar AA, et al. Identification of ?-Caryophyllene (BCP) in Aceh patchouli essential oil (PEO) using gas chromatography-mass pectrophotometry (GC-MS). InIOP Conference Series: Earth and Environmental Science 2021;667(1):012032. https://doi.org/10.1088/1755-1315/667/1/012032
Borges F, Roleira F, Milhazes N, et al. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Current Medicinal Chemistry 2005;12(8):887-916. https://doi.org/10.2174/0929867053507315

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Electronic Journal of Biotechnology